Abstract
Introduction: Daratumumab (DARA) is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor and immunomodulatory mechanism of action. DARA is approved as monotherapy or in combination with standard-of-care regimens for the treatment of relapsed or refractory multiple myeloma (RRMM). In the primary analysis (median follow-up, 16.9 months) of the phase 3 APOLLO study (NCT03180736), the addition of DARA to pomalidomide and dexamethasone (D-Pd) showed a significant progression-free survival (PFS) improvement over pomalidomide and dexamethasone (Pd) alone in patients (pts) with RRMM (Dimopoulos MA, et al. Lancet Oncol. 2021;22[6]:801-812). Here, we report results from the APOLLO study, including outcomes of pts refractory to lenalidomide based on the last dose of lenalidomide received.
Methods: Eligible pts were ≥18 years of age, had RRMM, had received ≥1 prior line of therapy (including both a proteosome inhibitor and lenalidomide), and had responded to prior treatment; pts with only 1 prior line of therapy were required to be refractory to lenalidomide. Pts were randomized 1:1 to Pd ± DARA, stratified via International Staging System disease stage (I vs II vs III) and number of lines of prior therapy (1 vs 2-3 vs ≥4). All pts received 28-day cycles of pomalidomide (4 mg PO daily on Days 1-21) and dexamethasone (40 mg [20 mg for pts ≥75 years of age], PO daily on Days 1, 8, 15, and 22). Pts in the D-Pd group received DARA subcutaneously (DARA SC; 1,800 mg; co-formulated with recombinant human hyaluronidase PH20 [rHuPH20; ENHANZE ® drug delivery technology, Halozyme, Inc.]) or, prior to a protocol amendment, intravenously (DARA IV; 16 mg/kg; n=7) weekly in Cycles 1-2, every 2 weeks in Cycles 3-6, and every 4 weeks thereafter. Pts receiving DARA IV were allowed to switch to DARA SC starting on Day 1 of Cycle 3 or later. All pts were treated until disease progression or unacceptable toxicity. The primary endpoint was PFS.
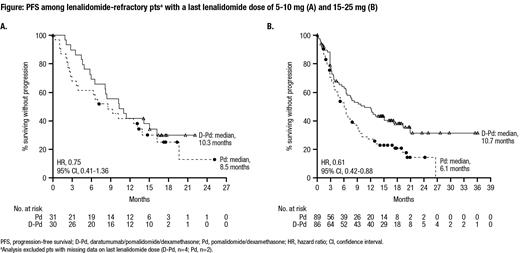
Results: In total, 304 pts were randomized (D-Pd, n=151; Pd, n=153). At the time of randomization, 120 (79.5%) pts in the D-Pd group and 122 (79.7%) pts in the Pd group were refractory to lenalidomide. Among pts refractory to lenalidomide, the last dose of lenalidomide received was 5-10 mg in 30 (25.0%) pts in the D-Pd group and 31 (25.4%) pts in the Pd group and was 15-25 mg in 86 (71.7%) pts in the D-Pd group and 89 (73.0%) pts in the Pd group. With a median follow-up of 16.9 months, the median PFS in the overall population was 12.4 months in the D-Pd group versus 6.9 months in the Pd group (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.47-0.85; P=0.0018); the estimated 18-month PFS rate was 42.1% for the D-Pd group and 25.5% for the Pd group. Among pts refractory to lenalidomide, the median PFS was 9.9 months in the D-Pd group and 6.5 months in the Pd group (HR, 0.66; 95% CI, 0.49-0.90). For lenalidomide-refractory pts who last received a dose of 5-10 mg lenalidomide, the median PFS was 10.3 months for the D-Pd group and 8.5 months for the Pd group (HR, 0.75; 95% CI, 0.41-1.36); for lenalidomide-refractory pts who last received a dose of 15-25 mg lenalidomide, the median PFS was 10.7 months for the D-Pd group and 6.1 months for the Pd group (HR, 0.61; 95% CI, 0.42-0.88) (Figure). Grade 3/4 treatment-emergent adverse events occurred in 130 (87.3%) pts in the D-Pd group and 123 (82.0%) pts in the Pd group; the most frequently reported (≥15% in both groups) were neutropenia (67.8% vs 50.7%), thrombocytopenia (17.5% vs 18.0%), and anemia (16.8% vs 21.3%). Grade 3/4 pneumonia occurred in 20 (13.4%) pts in the D-Pd group and 10 (6.7%) of pts in the Pd group. Serious adverse events occurred in 75 (50.3%) pts in the D-Pd group and 59 (39.3%) pts in the Pd group.
Conclusion: The addition of DARA to Pd provided a PFS benefit in the overall pt population and among pts refractory to lenalidomide. The safety profile observed in the APOLLO trial was consistent with previous reports for DARA SC and Pd. Additional analyses for lenalidomide-refractory pts, as well as efficacy and safety data for the overall population at longer follow up, will be presented.
Sonneveld: Celgene/BMS: Consultancy, Honoraria, Research Funding; SkylineDx: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding. Terpos: Celgene: Consultancy, Honoraria, Research Funding; Genesis: Consultancy, Honoraria, Research Funding; GSK: Honoraria, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; BMS: Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Boccadoro: Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol-Myers Squibb, and AbbVie: Honoraria; Janssen and GSK: Membership on an entity's Board of Directors or advisory committees; Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol-Myers Squibb, and Mundipharma: Research Funding. Delimpasi: Takeda: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Beksac: Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Katodritou: GSK, Amgen, Karyopharm, Abbvie, Janssen-Cilag, Genesis Pharma, Sanofi: Honoraria, Research Funding. Moreau: Celgene BMS: Honoraria; Sanofi: Honoraria; Oncopeptides: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Abbvie: Honoraria. Symeonidis: Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi/Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Demo: Research Funding; WinMedica: Research Funding; GenesisPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Bila: Janssen, Takeda, AMGEN: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Oriol: Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees. Mateos: Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Oncopeptides: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Honoraria; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees. Einsele: Janssen, Celgene/BMS, Amgen, GSK, Sanofi: Consultancy, Honoraria, Research Funding. Orfanidis: Health Data Specialists: Current holder of individual stocks in a privately-held company. Kampfenkel: Janssen: Current Employment. Qiu: Janssen: Current Employment. Amin: Janssen: Current Employment, Current equity holder in publicly-traded company. Kosh: Janssen: Current Employment. Tran: Janssen: Current Employment, Current equity holder in publicly-traded company. Carson: Janssen: Current Employment. Dimopoulos: Beigene: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Amgen: Honoraria; BMS: Honoraria.
The regimen is approved for patients who have received at least two prior therapies, whereas the APOLLO study included patients who have received at least 1 prior therapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal